MORC2-Related Disorder (M2RD)
Overview
MORC2 Related Disorder (M2RD) encompasses a spectrum of autosomal dominant disorders that primarily affect the central and/or peripheral nervous systems [1, 2, 4, 8]. M2RD can present from infancy to adulthood, with a broad range of phenotypes that tend to cluster into several distinct syndromes. The unifying feature of M2RD is a pathogenic variant in the gene MORC2. MORC2 is not included in many neurological and neuromuscular genetic test panels, though this may change as M2RD becomes increasingly recognized. Thus, the genetic diagnosis of M2RD is usually made via whole exome sequencing (WES) or whole genome sequencing (WGS), though this may change over time as genetic testing technologies and services evolve. The diagnosis of M2RD should be considered in individuals with certain genetically unsolved clinical syndromes such as polyneuropathy, non-5q spinal muscular atrophy, or features resembling Cockayne Syndrome.
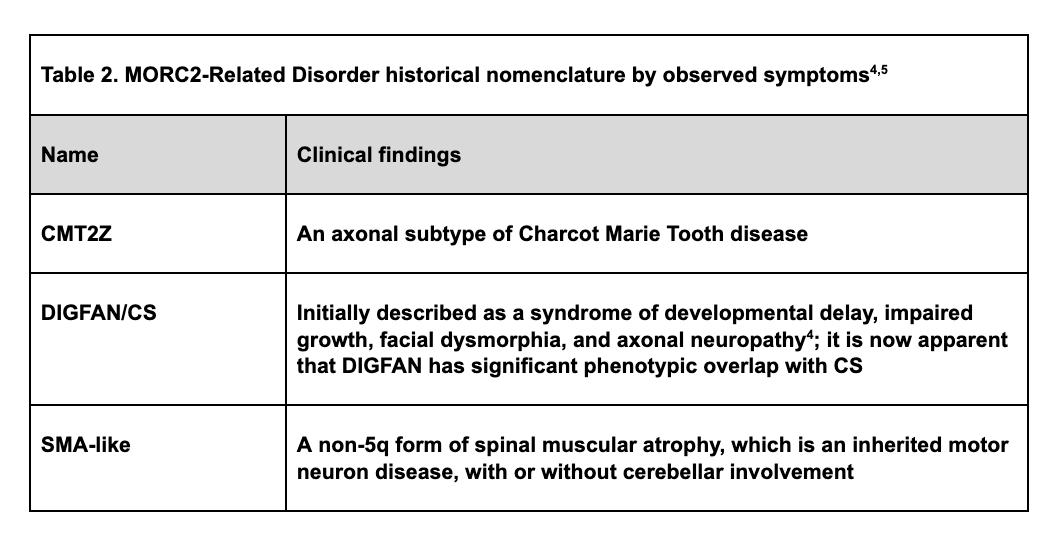
Due to complex genotype-phenotype correlations [16], there has been some inconsistency in nomenclature for the diseases associated with pathogenic variants in MORC2. Such associated diseases include Charcot-Marie-Tooth disease type 2Z (CMT2Z); a rare variant of spinal muscular atrophy (SMA); a syndrome of developmental delay, impaired growth, facial dysmorphia, and axonal neuropathy (DIGFAN); and Cockayne Syndrome [4, 5]. DIGFAN and Cockayne Syndrome have very similar phenotypes and may be two names for the same clinical syndrome. This inconsistency has resulted prolonged diagnostic odysseys, misdiagnoses, and delays in the medical characterization and downstream therapeutic support of patients living with M2RD. Specific locations of pathogenic variants affecting different domains of the encoded protein, along with other factors, account for the variable clinical presentations observed in M2RD [8, 16]. Genetic testing such as WES or WGS should be considered in all patients with undiagnosed neurodevelopmental and neuromuscular disorders to maximize the likelihood of arriving at an accurate genetic diagnosis. A genetic diagnosis of M2RD early in the patient journey will help researchers understand this rare disorder better and further our progress towards improved clinical management and the development of novel therapies.
Pathophysiology
M2RD is caused by dominant pathogenic variants in the MORC2 gene. Such variants generate dysfunctional MORC2 protein, which in turn impairs the regulation of gene transcription and silencing [8, 1]. MORC2 is one of a quartet of MORC proteins: MORC1, MORC2, MORC3, and MORC4 [3, 16]. To date, known pathogenic variants causing M2RD are located in the ATPase region of the gene which contains the GHKL, CC1, and S5 domains [2, 3, 5]. Aberrant MORC2 ATPase activity perturbs ATP hydrolysis [16]. A pattern that is emerging from the cases of M2RD published to date is the association of variants affecting specific ATPase protein domains with specific phenotypes [5]. Variants affecting the GHKL domain are associated with a more severe phenotype [4]. Individuals with variants affecting the S5 and CC1 regions have less uniform symptom patterns that often include peripheral neuropathy [4]. Overall, symptom severity is highly variable with individuals harboring the same variant displaying different phenotypes [5]. While the exact mechanism behind this variability is not completely understood, MORC2 has been shown to be a key requirement for normal activity of the HUSH complex which regulates epigenetic silencing, suggesting that multifactorial influences on epigenetic changes may be in part responsible for the clinical variability of M2RD [18].
M2RD with pathogenic variants affecting the GHKL domain generally presents in infancy or early childhood, manifesting as a syndrome that was initially described as developmental delay, impaired growth, facial dysmorphia, and axonal neuropathy (DIGFAN) [12] and now recognized as meeting clinical criteria for Cockayne Syndrome (CS) [4]. Pathogenic variants affecting the CC1 and S5 domains show less consistency in age of onset and severity, with onset ranging from childhood to adulthood and multiple associated phenotypes including CMT2Z, SMA, and DIGFAN/Cockayne syndrome. Some individuals develop distal weakness that spreads proximally and may be accompanied by central nervous system involvement (Table 1) [16].
Figure 1. Diagram of the MORC2 protein illustrating details of the ATPase region (amino acids 1-469) where pathogenic variants leading to M2RD have been reported to date. Pathogenic variants in the ATPase region are thought to disrupt the process of protein dimerization and ATP hydrolysis needed for chromatin remodeling and gene silencing [16].
MORC2 plays a critical role in DNA repair , chromatin regulation, adipogenesis, lipid homeostasis, and epigenetic silencing via its interaction with the HUSH complex [2, 3, 5-7]. While there is an incomplete understanding of how pathogenic variants in MORC2 drive specific neurodegenerative patterns in the peripheral and central nervous systems, it has been observed that MORC2 is ubiquitously expressed in the brain which implies a crucial role for its function in neural tissue [5, 8].
The relation of tissue MORC2 overexpression in malignancies has been widely described and is associated with many cancers (lung, kidney, prostate, oesophagus, breast, liver, colon, stomach, ovarian, pancreas, skin, endometrium, naso-pharynx, and bladder), and may play a causal role in tumorigenesis and metastasis [3, 6, 8-11]. MORC2 overexpression is implicated in radioresistance, chemoresistance, endocrine resistance and in cancer progression, with high levels of MORC2 associated with poor prognosis in breast and gastric cancers [3, 9, 10]. Single nucleotide variants, deletions and insertions have all been observed [25] though to date no clear pattern has been reported related to the location of the mutation on the gene or whether MORC2 mutations in cancer are tissue specific only or globally expressed . Whether there is an association between M2RD and either increased or decreased risk of cancer has not yet been determined.
Advances in genomics have led to the discovery of the M2RD family of Mendelian disorders (Table 2) [12-14]. An urgent need exists to examine the natural history of M2RD and establish outcome measures in order to further our understanding of these disorders and evaluate novel therapeutic approaches.
Signs and symptoms
A common thread that runs through all known forms of M2RD is neuro-developmental impairment, with significant phenotypic variability between and sometimes within subtypes [4]. Age of onset ranges from infancy to the fourth decade of life [15], raising the possibility that there are individuals of all ages with M2RD whose diagnoses remain unrecognized or unconfirmed.
The presence of a sensorimotor neuropathy with varying muscle tone and weakness is widespread in M2RD [4, 13]. Weakness may extend to proximal muscles over time [5]. Reported clinical, neuromuscular and imaging findings are detailed below.
Features Associated with M2RD
Clinical
Chronic constipation [4]
Facial dysmorphia1
Failure to meet motor developmental milestones (walking and sitting) [22]
Frequent dental caries [4]
Global developmental delay [1, 5]
Hearing loss (sensorineural or mixed) [12]
Intellectual delay [1]
Nocturnal hypoventilation [24]
Precocious puberty [12]
Retinal dystrophy [4]
Scoliosis and contracture [4]
Short stature [4]
Thyroid dysfunction [4]
Vocal cord paralysis [23]
Neuromuscular
Abnormal deep tendon reflexes (may be increased or decreased) [4, 22, 23]
Cerebellar ataxia [12-14]
Distal muscle weakness [23]
Gait disturbance, inability to achieve independent ambulation [15, 18]
Respiratory muscle weakness [22]
Tremor [1, 4]
Hypotonia [22]
Pyramidal signs [23]
Brain imaging and Cranial
Cerebellar and vermian atrophy [4, 14]
Increased T2 signal at the basal ganglia and parietal regions [4]
Optic nerve atrophy [23]
Microcephaly [4]
Causes
M2RD is associated with autosomal dominant pathogenic variants in the MORC2 gene. To date, pathogenic variants have been reported in the GHKL, CC1 and S5 domains, which are responsible for ATPase activity of the MORC2 protein [16]. A study of transgenic mice that carry a heterozygous single nucleotide variant in the GHKL domain of MORC2 found an absence of homozygous mutant pups, suggesting that homozygosity may be embryonic lethal and that at least some functional MORC2 protein is needed for foetal development and viability [21]. Reported pathogenic variants in MORC2 are almost exclusively de novo, meaning mutations were not inherited from parent to offspring. One pair of siblings with M2RD has been reported, potentially related to mosaicism of one of the parents as neither parent was found to carry the variant [4]. The severity of symptoms appears to be associated with various factors, including the location of the pathogenic variant on the MORC2 gene. Other factors that may be involved but have not been confirmed include epigenetic effects, gene-gene interactions, modifying genes, and the quality and appropriateness of supportive care.
Diagnosis
As with other Mendelian disorders, confirmation of the diagnosis of M2RD requires genetic testing. Because M2RD has been identified in the presence of neuromuscular abnormalities [4], clinical evaluations accompanied by genetic testing, including whole exome sequencing (WES) or whole genome sequencing (WGS) when indicated, would be consistent with the 2016 AANEM consensus statement on genetic testing for neuromuscular disorders [15].
Clinical evaluations can include electrodiagnostic studies (EDX) to confirm the presence of a sensorimotor axonal neuropathy. EDX includes nerve conduction studies (NCS), which, for an axonal neuropathy, would show normal or diminished action potential amplitudes in the setting of normal conduction velocities [2]. A second component of EDX is electromyography (EMG), which in the setting of axonal neuropathy would typically show signs of denervation and/or chronic reinnervation.
Brain MRI may show cerebellar and/or vermian atrophy in some affected individuals [4].
Sural nerve biopsy is now a less commonly performed diagnostic procedure due to its invasiveness and the increasing yield of genetic testing [20]. In M2RD, sural nerve biopsy has been reported to show axonal degeneration without hypertrophic features, and demyelination with onion bulb formation is not seen or is minimal [17]. The cost/benefit of all invasive testing should be carefully considered, particularly given the need for confirmatory genetic testing in M2RD.
In view of its variable clinical features and ultra-rare occurrence, M2RD may not specifically appear on a differential diagnosis even when an individual presents with symptoms and signs of M2RD. Thus, genetic test panels for sensorimotor neuropathy, non-5q spinal muscular atrophy, and DNA repair disorder should include MORC2 whenever possible [4]. When genetic test panels do not include MORC2 for a specific patient who has some features of M2RD, WES and/or WGS should be considered as those test modalities will likely capture pathogenic variants in MORC2. Given the relatively recent discovery of disease associations with MORC2 and the barriers to patient access to WES and WGS in many clinical settings, M2RD is likely to be significantly underreported.
Progression
Little is currently known about the disease progression of M2RD due to the limited number of published cases and the cross-sectional nature of these studies. Some features of M2RD have been reported to progress while others remain stable for at least a period of time. Individuals who present later in life may experience worsening distal limb weakness leading to diagnosis [2]. Due to the critical function of MORC2 in genomic DNA homeostasis, it progression of some clinical features is likely, though little is currently known on what compensatory mechanisms may successfully slow or arrest clinical progression. There is a significant need for longitudinal and natural history studies on M2RD patients to better understand how M2RD changes over time and to identify the risk factors for progression and associated symptoms .
Treatment
Due to the phenotypic variability of M2RD and its novel status, treatment regimens are currently supportive, empirical and individualised to the needs of each patient. A multi-disciplinary approach from a team of specialists is required to systematically and comprehensively formulate a management plan for each individual. Depending on the needs of the individual patient, specialists who could contribute to patient management either before or after diagnosis should include a paediatrician, paediatric neurologist, physiotherapist, occupational therapist, and geneticist. To enhance quality of life and assist in overall clinical management, additional support should be considered from gastroenterology, dietary sciences, speech therapy, physical medicine & rehabilitation, orthopaedics, and/or psychology/ behavioral health. Other specialty input may also be warranted in special circumstances. For example, if a peripheral neuropathy impacts upper and/or lower airway function, a pulmonologist’s input would be valuable [22].
When available and eligibility criteria are met, early developmental intervention programs are helpful in optimizing an affected individual’s developmental potential during early childhood. Additional social and/or vocational services including individualized education programs are impactful during school-age years and adolescence. Psychosocial support for the entire family is imperative.
Physiotherapists, physical medicine & rehabilitation physicians, and/or orthopedic surgeons can offer individualised care of contractures. This may include exercises (administered directly or at the home by parents), orthotic devices and, typically as a last resort, surgical interventions. The risks and benefits of invasive surgical procedures should be considered in the context of the long-term course of M2RD. For example, the beneficial effects of heel cord release procedures may attenuate over time.
Pain management should also be considered given the impactful experience of cramps and contractures. This is especially important to consider in the non-verbal and/or younger patient where communication may be difficult. When available, non-pharmacologic strategies for pain management such as massage therapy can serve as valuable add-ons.
Looking Ahead
A key step in developing novel therapies and clinical management guidelines for M2RD is to ensure confirmatory genetic diagnosis of all affected individuals and help patients and caregivers become aware of clinical research opportunities. Currently, the understanding of M2RD is in its infancy with less than 100 patients having participated in clinical research globally. A larger population of diagnosed patients participating in research will drive advancement in the clinical and physiologic characterization of M2RD which will help scientists and medical providers determine how best to treat patients with the current tools available as well as what opportunities may be availably for gene therapies once M2RD has been well defined.
References
1. Maguina, M., Kang, P. B., Tsai, A. C., & Pacak, C. A. (2023). Peripheral neuropathies associated with DNA repair disorders. Muscle & nerve, 67(2), 101-110.
2. Jacquier, A., Ribault, S., Mendes, M., Lacoste, N., Risson, V., Carras, J., ... & Schaeffer, L. (2022). Expanding the phenotypic variability of MORC2 gene mutations: From Charcot‐Marie‐Tooth disease to late‐onset pure motor neuropathy. Human Mutation, 43(12), 1898-1908.
3. Wang, H., Zhang, L., Luo, Q., Liu, J., & Wang, G. (2021). MORC protein family-related signature within human disease and cancer. Cell Death & Disease, 12(12), 1112.
4. Stafki, S. A., Turner, J., Littel, H. R., Bruels, C. C., Truong, D., Knirsch, U., ... & Kang, P. B. (2023). The Spectrum of MORC2-Related Disorders: A Potential Link to Cockayne Syndrome. Pediatric neurology, 141, 79-86.
5. Jacquier, A., Roubille, S., Lomonte, P., & Schaeffer, L. (2022). Microrchidia CW-Type Zinc Finger 2, a Chromatin Modifier in a Spectrum of Peripheral Neuropathies. Frontiers in Cellular Neuroscience, 16.
6. Guddeti, R. K., Chutani, N., & Pakala, S. B. (2021). MORC2 interactome: its involvement in metabolism and cancer. Biophysical Reviews, 13(4), 507-514.
7. Sánchez-Solana, B., Li, D. Q., & Kumar, R. (2014). Cytosolic functions of MORC2 in lipogenesis and adipogenesis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1843(2), 316-326.
8. Tchasovnikarova, I. A., Timms, R. T., Douse, C. H., Roberts, R. C., Dougan, G., Kingston, R. E., ... & Lehner, P. J. (2017). Hyperactivation of HUSH complex function by Charcot–Marie–Tooth disease mutation in MORC2. Nature genetics, 49(7), 1035-1044.
9. Liu, H. Y., Liu, Y. Y., Yang, F., Zhang, L., Zhang, F. L., Hu, X., ... & Li, D. Q. (2020). Acetylation of MORC2 by NAT10 regulates cell-cycle checkpoint control and resistance to DNA-damaging chemotherapy and radiotherapy in breast cancer. Nucleic acids research, 48(7), 3638-3656.
10. Pan, Z., Ding, Q., Guo, Q., Guo, Y., Wu, L., Wu, L., ... & Zhou, F. (2018). MORC2, a novel oncogene, is upregulated in liver cancer and contributes to proliferation, metastasis and chemoresistance. International Journal of Oncology, 53(1), 59-72.
11. Ding, Q. S., Zhang, L., Wang, B. C., Zeng, Z., Zou, X. Q., Cao, P. B., ... & Zhou, F. X. (2018). Aberrant high expression level of MORC2 is a common character in multiple cancers. Human Pathology, 76, 58-67.
12. Sacoto, M. J. G., Tchasovnikarova, I. A., Torti, E., Forster, C., Andrew, E. H., Anselm, I., ... & Juusola, J. (2020). De novo variants in the ATPase module of MORC2 cause a neurodevelopmental disorder with growth retardation and variable craniofacial dysmorphism. The American Journal of Human Genetics, 107(2), 352-363.
13. Schottmann, G., Wagner, C., Seifert, F., Stenzel, W., and Schuelke, M. (2016). MORC2 mutation causes severe spinal muscular atrophy-phenotype, cerebellar atrophy, and diaphragmatic paralysis. Brain 139:e70. doi: 10.1093/brain/aww252
14. Zanni, G., Nardella, M., Barresi, S., Bellacchio, E., Niceta, M., Ciolfi, A., et al. (2017). De novo p.T362R mutation in MORC2 causes early onset cerebellar ataxia, axonal polyneuropathy and nocturnal hypoventilation. Brain 140:e34. doi: 10.1093/brain/awx083
15. Semplicini, C., Ollagnon-Roman, E., Leonard-Louis, S., Piguet-Lacroix, G., Silvestre, M., Latour, P., et al. (2017). High intra-familiar clinical variability in MORC2 mutated CMT2 patients. Brain 140:e21. doi: 10.1093/brain/awx019
16. Douse, C. H., Bloor, S., Liu, Y., Shamin, M., Tchasovnikarova, I. A., Timms, R. T., ... & Modis, Y. (2018). Neuropathic MORC2 mutations perturb GHKL ATPase dimerization dynamics and epigenetic silencing by multiple structural mechanisms. Nature communications, 9(1), 651.
17. Keller, M. P., & Chance, P. F. (1999). Inherited peripheral neuropathy. In Seminars in neurology (Vol. 19, No. 04, pp. 353-362). © 1999 by Thieme Medical Publishers, Inc..
18. Sevilla, T., Lupo, V., Martínez-Rubio, D., Sancho, P., Sivera, R., Chumillas, M. J., ... & Espinos, C. (2016). Mutations in the MORC2 gene cause axonal Charcot–Marie–Tooth disease. Brain, 139(1), 62-72.
19. Kassardjian, C. D., Amato, A. A., Boon, A. J., Childers, M. K., Klein, C. J., & AANEM Professional Practice Committee. (2016). The utility of genetic testing in neuromuscular disease: a consensus statement from the AANEM on the clinical utility of genetic testing in diagnosis of neuromuscular disease. Muscle & nerve, 54(6), 1007-1009.
20. Prada, V., Massucco, S., Venturi, C., Geroldi, A., Bellone, E., Mandich, P., ... & Schenone, A. (2019). Diagnostic value of sural nerve biopsy: retrospective analysis of clinical cases from 1981 to 2017. Frontiers in Neurology, 10, 1218.
21. Lee, G. S., Kwak, G., Bae, J. H., Han, J. P., Nam, S. H., Lee, J. H., ... & Yeom, S. C. (2021). Morc2a p. S87L mutant mice develop peripheral and central neuropathies associated with neuronal DNA damage and apoptosis. Disease Models & Mechanisms, 14(10), dmm049123.
22. Stettner, G., Knirsch, U., Berger, W., Graf, U., Hendriks, B., Seidl, R., ... & Weiss, S. (2019). EP. 113Infantile-onset CMT2Z is caused by two MORC2 gene mutations and is associated with a distinct phenotype. Neuromuscular Disorders, 29, S200-S201.
23. Kwon, H. M., & Choi, B. O. (2021). Analyzing clinical and genetic aspects of axonal Charcot-Marie-Tooth disease. Journal of Genetic Medicine, 18(2), 83-93.
24 Albulym, O. M., Kennerson, M. L., Harms, M. B., Drew, A. P., Siddell, A. H., Auer‐Grumbach, M., ... & Nicholson, G. A. (2016). MORC 2 mutations cause axonal Charcot Marie Tooth disease with pyramidal signs. Annals of neurology, 79(3), 419-427.
25. COSMIC catalogue of somatic mutation in cancer. Database accessed 18 April 2023. https://cancer.sanger.ac.uk/cosmic/search?q=MORC2